Background and Introduction
In this guide
In this guideOn this page
Skip the menu of subheadings on this page.Background
1. The Scientific Advisory Committee on Nutrition (SACN) last considered maternal diet and nutrition in relation to offspring health, in its reports on ‘The influence of maternal, fetal and child nutrition on the development of chronic disease in later life’ (SACN, 2011) and on ‘Feeding in the first year of life’ (SACN, 2018). In the latter report, the impact of breastfeeding on maternal health was also considered. In 2019, SACN agreed to conduct a risk assessment on nutrition and maternal health focusing on maternal outcomes during pregnancy, childbirth and up to 24 months after delivery; this would include the effects of chemical contaminants and excess nutrients in the diet.
2. SACN agreed that, where appropriate, other expert Committees would be consulted and asked to complete relevant risk assessments e.g., in the area of food safety advice, and this was referred to the Committee on the Toxicity of Chemicals in Food, Consumer Products and the Environment (COT). The subject was initially discussed by the COT during the horizon scanning item at the January 2020 meeting with a scoping paper being presented to the Committee in July 2020. This included background information on a provisional list of chemicals proposed by SACN. It was noted that this was subject to change following discussion by COT, who would be guiding the toxicological risk assessment process: candidate chemicals or chemical classes can be added or removed as the COT considered appropriate. The list was brought back to the COT with additional information in September 2020. Following a discussion at the COT meeting in September 2020, it was agreed that papers on a number of components should be prioritised. Of the groups prioritised was ergot alkaloids (EAs) and the following paper provides the advice of the COT on whether exposure to EAs would pose a risk to maternal health.
Introduction
3. EAs are secondary metabolites produced by the fungi families Clavicipitaceae and Trichocomaceae, with Claviceps purpurea being the most widespread EA producing species in Europe. Infection by these fungi can affect more than 400 plant species, including some economically important cereal grains such as rye, wheat, triticale, barley, millet and oats (Agriopoulou, 2021).
4. The biological effects of EAs have been known for centuries, including their traditional use in obstetrics. Consumption of contaminated grains, flour or bread caused severe epidemics of a condition known as Erysipelas or St. Anthony’s fire. Since the first systematic investigations in the 1900s, many natural EAs and their synthetic analogues have been used as pharmaceutical agents to treat central nervous system diseases (Tasker and Wipf, 2021).
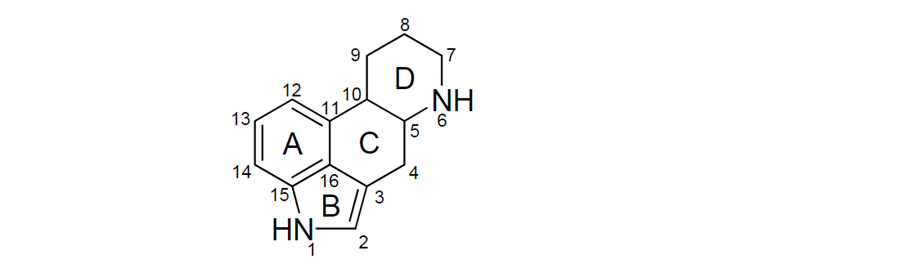
5. Most of the naturally occurring EAs contain a tetracyclic ergoline ring system (Figure 1) consisting of four fused rings with the N6 position carrying a methyl group, and a double bond at either C8,9 or at C9,10 (EFSA, 2012). There are 80 different naturally occurring EAs (Schiff, 2006). Based on their occurrence and the available toxicological data, the European Food Safety Authority (EFSA) considered six EAs in their risk assessment in 2005, namely ergotamine, ergocornine, α-ergocryptine, ergosine, ergocristine (peptide ergot alkaloids) and ergometrine (a lysergic acid amide). For Δ9,10-ergolenes the asymmetric centre at C8 (Figure 1) gives rise to two epimers, with a double bond at C9/10, β-Δ9,10-ergolenes (suffix -ine) and α-Δ9,10-isoergolenes (suffix -inine). While the -inine forms of EAs are considered biologically inactive, interconversion occurs frequently and hence EFSA included both forms of EAs (-ine and inine) in their assessment (EFSA, 2005, Tasker and Wipf, 2021).
Figure 1: Ergoline ring system including numbering and assignment of ring (EFSA, 2012).
6. A number of derivatives of EAs have been developed for use (or potential use) as pharmacological agents. Bromocriptine is a synthetic ergoline derivate and is used in the treatment of Parkinson’s disease and pituitary tumours (Hardman et al., 2001). Lysergic acid diethylamide (LSD) is a semi-synthetic derivative of the EA-family, first produced in the 1950s. This illegal drug has worldwide recreational (ab)use and is known to cause psychoactive effects. Ergometrine is a derivate of lysergic acid (LA) used primarily in obstetrics.
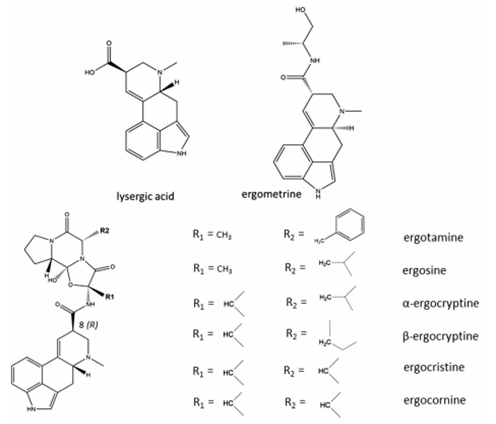
7. Other EAs considered in this evaluation were peptide alkaloids with a cyclized tripeptide as a substituent at C8 (EFSA, 2012, JECFA, 2023). The chemical structures of the most prevalent EAs are illustrated in Figure 2.
Figure 2: Chemical structures of the most prevalent EAs (JECFA, 2023).